First Law of thermodynamics
The first law of thermodynamics is based on the principle of conservation of energy and it states that whenever heat is added to a
system, the heat will transform to some other form of energy in the same
amount.
In this lesson, you will learn to understand the equation Q = ∆E - W or Q = ∆E + W
A system could be anything where heat is being added to include the following:
- A steam engine that will power a train or a vehicle
- A steam turbine that will generate electricity
- Your body
- A pressure cooker
What happens then when you add heat to a system?
This heat can either increase the internal energy of the system if it does not leave the system, do external work if it leaves the system or do a combination of both.
We can then restate the first law of thermodynamics as shown below.
Heat added to a system = increase in internal energy of the system + external work done by the system.
An important thing to keep in mind about the first law of thermodynamics!
Keep in mind that if the system does no work, the internal energy will keep on increasing. By the same token, the pressure inside the system will increase as well. This may become very dangerous since if the system cannot tolerate the constant increase of pressure, it may explode.
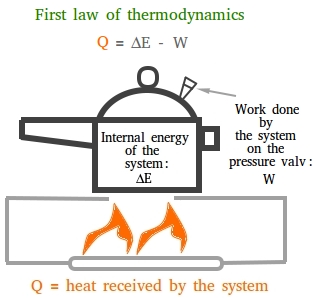
Imagine a pressure cooker built in a way so that no steam will be allowed to escape and you put it on a stove and leave the burner on! The internal energy will get higher and higher and this may eventually create an explosion.
That is why a pressure cooker is built with a safety feature. This safety feature is usually a spring-loaded valve that will allow some of the steam to escape.
As the steam is coming out, it does some work on the valve by raising the weight of the valve. Thus, heat has been converted to mechanical energy.
Mathematical formulation of the first law of thermodynamics
Let Q = the net quantity of heat the system received
Let ΔE or ΔU = increase in internal energy or generally speaking change in internal energy.Let W = work done by the system. We can then rewrite the 1st law of thermodynamics as shown below.
Q = ΔE + wSubtract w from both sides
Q - w = ΔE + w - w
ΔE = Q - w
The formula above represents work done by the system.
The work done on the system is the negative of the work done by the system.
The figure below mentioned earlier shows the first law of thermodynamics when a pressure cooker is heated.

ΔE = Q + w
Why the work done on the system is always the negative of the work done by the system
Why ΔE = Q + w when work is done on the system?When work is done on the system, the internal energy of the system tends to increase.
However, if we don't turn Q - w into Q + w, we will end up with a contradiction as shown below
Suppose ΔE = Q - w
If Q = 0, ΔE = -w
The negative number next to w shows decrease not increase of internal energy.
It is like going from 8 joules to 6. Change in energy is 6 - 8 = -2
Since -2 is a negative number, it shows that the energy has been decreased.
By the same token, -w shows that the energy has been decreased.
Heat is not the only way to create change in internal energy
Adding heat is not the only to increase or decrease the internal energy of a system. Let Q = 0
Work can be done on a system by compressing it.
ΔE = 0 + wΔE = w
In this case, the work will cause an increase in energy and thus an increase in temperature without using any heat input.
An example of this is when you are using a bicycle pump. When pumping
the handle, you are doing work on the system. If you touch the pump, you
will see that it is a little hot although no heat was added to the
pump. This is because you increase the internal energy of the pump while
you were doing work on the system.
ΔE = -w
The internal energy in this case will decrease.
Thermodynamic systems
There are three types of thermodynamic systems
- Open system
- Closed system
- Isolated system
Open system
In an open system, the transfer of matter and energy such as work or heat transfer is allowed with its surroundings. Suppose you are cooking meat in a pot or saucepan and you do not use any lid to cover the saucepan. This is an open system since matter in the meat can get into the air and matter in the air can also get into the meat. Refrigerators are also examples of open systems.
Closed system
In a closed system, the transfer of matter is not allowed but energy transfer such as work or heat is allowed with its surroundings. A pressure cooker is a good example of a closed system. Suppose you are trying to cook meat in a pressure cooker. There is a transfer of energy and the internal energy of the pressure cooker will keep on increasing so the meat can cook faster and become tender. However, because a pressure is sealed, no transfer of matter will happen with it surroundings. Air conditioners are also examples of closed systems.
Isolated system
In an isolated system, no exchange of matter or energy such as heat or work is allowed with its surroundings. A thermos bottle is a good example of isolated system. The thermos is made in a specific way to prevent heat from entering or escape the thermos. The rigid boundary of the thermos also prevents the transfer of matter.