Second law of thermodynamics
In this lesson, you will know what the second law of thermodynamics is about and how this law is used in heat engines.
The second law of thermodynamics states that heat will flow from hot to
cold. Heat will never flow from a cold object to a hot object unless we
use some external effort such as a heat pump.
Why does your house get cold in winter? It is not because cold air from the outside got inside your house. It is because the hot air inside your house went outside.
During the summer though, heat from the outside gets inside your cool house and make your house hot.
Second law of thermodynamics and heat engines
A heat engine is a device that can change internal energy or heat into mechanical work. A heat engine however cannot completely change heat into work.
Only a portion of the heat will be converted into mechanical work and whatever portion of the heat that is left will be lost in the process.
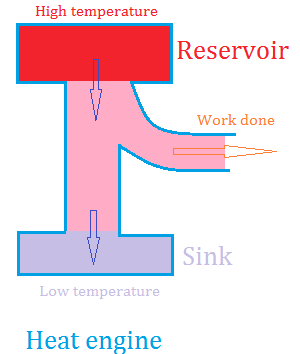
The process of converting heat to work by a heat engine can be outlined as follow.
1. Get heat from a heated source or reservoir of higher temperature and send it to an object.
2. Make the object do mechanical work by using some of the heat.
3. Sent the rest of the heat to a reservoir where the temperature is lower. This is usually called a sink.
The diagram below illustrates the process.
In a car engine, the burning fuel in the combustion chamber is the hot reservoir.
Some of the heat generated will do mechanical work.
The rest of the heat or internal energy will pass through the exhaust.
Because some of the heat was not used, you may notice that the engine and the exhaust are hot.
Since some of the heat is lost in the process, it is useful to know and
calculate the efficiency of an engine. In other words, we are trying to
determine how much of the input will end up doing useful work.
French engineer Sadi Carnot discovered a useful formula.
Let TR be the temperature of the hot source or reservoir.Let TS be the temperature of the cold reservoir.
Carnot found that the efficiency is the ratio of "the difference in temperature between heated source and sink" to "temperature of heated source"
For example, suppose the temperature of the source is 300° and the temperature of the sink is 200°
This means that only 33.33% of the heat can be converted into work. The rest will go to waste.
You can improve efficiency by increasing the temperature of the source.
Say the temperature of the source is 600° instead of 300°
Now 66.66% of the heat will get some work done!