Specific heat capacity
Specific heat capacity, also called specific heat or thermal
capacity, is defined as the amount of heat needed to raise the
temperature of a substance by 1 degree. Take a look at the table below to see some examples.
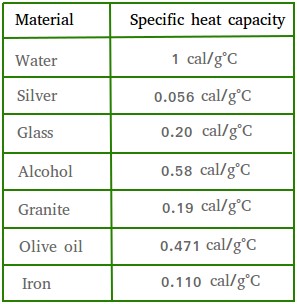
Notice that the amount of heat needed will vary and really depends on the substance.
For example, it takes a pot of water on the stove about 15 minutes to go from room temperature to its boiling point.
However, if you put an equal mass of silver on the same flame, it will take less than a minute to rise to the same temperature.
We say that silver has a lower specific heat capacity than water since silver will get hot much quicker than water.
A substance that has a high specific heat will remain hot for a longer period of time while a substance with a low specific heat will remain hot for a shorter period of time.
Specific heat capacity of aluminum foil
Many people use aluminum foil to cover their food while the food is cooking in the oven.
After you remove the food from the oven, did you notice that you can peel off the aluminum foil with your bare fingers?
The reason for this is because aluminum foil has a low specific heat.
It gets hot very fast in the oven. However, as soon as it comes out of the oven, it will cool down very fast.
Specific heat capacity of iron
You will get a different result if you put an iron tray in the oven.
You will then have to wait a long time before you can touch it. Again, it is because it takes longer to get hot and longer to cool down as well.
Recall that 1 calorie is the amount of heat required to raise the temperature of 1 gram of water by 1 degree celsius.
That is why we say that the specific heat of water is 1 cal/g°CAs you can see, the unit of specific heat is cal/g°C (calories per gram °C)
The specific heat of a substance does not change. The amount of heat needed then will depend on the mass of the substance and the temperature.
What is the amount of heat needed for 4 grams of water?
Since 1 gram requires 1 calory, does it make sense to say that 4 grams will require 4 calories? Yes, it does!
Mathematically, this can be expressed as4 g × 1 cal/g × 1 °C = 4 calories.
Notice that multiplication is the best way to express this relationship between specific heat, mass, and temperature.
What is the amount of heat needed to raise 4 grams of water by 3 degree Celsius?
1 degree Celsius requires 4 calories, so 3 degrees Celsius will require 12 calories.
Mathematically, this can be expressed as4 g × 1 cal/g × 3 °C = 12 calories.
Specific heat capacity formula
Q = mcΔT
- Q is the amount of heat
- m is the mass
- c is the specific heat capacity
- T is the change in temperature
What is the number of calories needed to raise the temperature of 3 liter of water by 20 degrees Celsius?
1 liter of water is equal to 1000 grams, so 3 liters of water will equal to 3000 grams. Now, just use the formula
Q = mcΔTQ = 3000 g × 1 cal/g × 20
Q = 60,000 calories.