States of matter
The four fundamental states of matter are solid, liquid, gas, and plasma. What is matter though?
Atoms are the building blocks of matter. In other words, when you put lots of atoms together, you end up with matter.
Still another way to define matter is to say that whenever you have volume and mass, you have matter. View this way, we can then say that matter is everywhere.
Examples of matter:
- A book is matter (A book has a mass and it has a volume)
- The human body is matter (The human body has a mass and it has a volume)
- The earth is matter (The earth has a mass and it has a volume)
The four states of matter
You are definitely familiar with solid, liquid and gas phases or states. However, you may not be familiar with the plasma state. Keep reading to see the explanations!
In all phases, the atoms are constantly in motion. You may find it hard to believe, but this fact was discovered by Scottish botanist Robert Brown.
He was studying grains of pollen under a microscope when he noticed that the spores were in constant state of agitation. Of course, at first he thought that the spores were some sort of living things. However, he discovered the exact same thing with dust particles.
The movement of particles big enough to be seen is called Brownian motion.
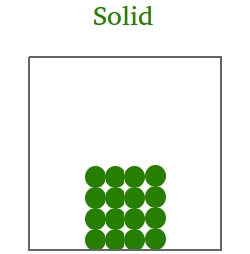
In the solid state,
- Atoms and molecules will vibrate about fixed positions.
- There is not much space between atoms and/or molecules.
- The solid will keep its shape.
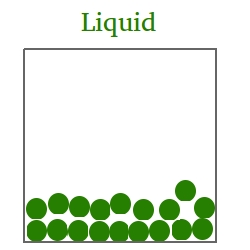
In the liquid state,
- Atoms and molecules will wander throughout the material at small speeds.
- There is more space between atoms and/or molecules.
- The liquid will not keep its shape, but take the shape of the container.
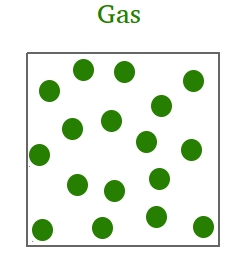
In the gaseous state,
- Atoms and molecules will wander randomly in the material at greater speed.
- There is lots of space between atoms and/or molecules.
- The gas will take the shape of the container.
To get to the plasma phase, you just have to heat gas at a high temperature. In this phase, matter has only positive ions after the electrons have been freed by heat.
The combination of positive ions and moving electrons is called plasma.
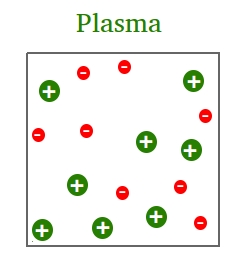
A great example is the sun. The sun has enough heat to cut loose the electrons from the hydrogen and helium that make up the sun.
Simply put, this combination of ions and moving electrons makes the sun shine bright in the sky!
Plasma TV and fluorescent lamp are other examples of the plasma phase.
All substances can go from one state to another by adding or removing heat.
A great example of this is water. If we heat ice, we get water. If we heat water, we get steam or gas.
The reverse happens too. When the air is cooled down to its dew point the water vapor in the air changes back into water. The water falls to the ground. This process is called condensation.
If you continue to lower the temperature of water, it turns back into ice.
States of matter quiz
Take the states of matter quiz below to see how well you know the 4
states of matter. After completing this quiz, you should be able to
quickly differentiate between the states of matter. You will not need to
use a paper and pencil to complete this quiz.
First, read this lesson about states of matter and then take this quiz.
Objective of the quiz:
- Know what matter is.
- Know the difference between matter and atom.
- Know some examples of matter
- Know what the four states of matter are
- Know when matter is in solid state
- Know when matter is in liquid state
- Know when matter is in gaseous state
- Know when matter is in plasma state
|
Test your knowledge with the quiz below: |