Thermal expansion of water and the reason that ice floats on the surface on water
The thermal expansion of water is different than most other liquids. With the exception of water, almost all liquids will expand with an increase in temperature.
However take a look at the graph below to see how water behaves as the temperature increases from 0 degree to 4 degrees Celsius. Keep in mind that at zero degree, water is in its solid form.
As the temperature increases from 0 degree to 4 degrees, we notice that the volume is decreasing.
At 4 degrees Celsius, a given amount of water has the smallest volume. Since that amount of water has the smallest volume, it has the greatest density.

Recall that the denser something is, the heavier that thing is. Therefore, at 4 degrees Celsius, water is the heaviest.
Now, what happens if the temperature is higher than 4 degrees?
If the temperature is bigger than 4 degrees, the volume of water will start increasing and as a result the water will be less dense.
If the temperature is between 0 degree and 4 degrees, the water will increase in volume and become less dense.
If the temperature is smaller than 0 degree, it will contract.
Thermal expansion of water and floating ice
Why does ice float on the surface of water as opposed to sinking to the bottom?
Recall that the greatest density of water happens at 4 degrees. This water that has a temperature of 4 degrees will settle at the bottom since it is the heaviest.
Ice forms with a temperature of 0 degree. Ice is less dense or lighter than water. Therefore, it settles on the surface of the water.
Here is the process
The water temperature of a pond is 20 degrees.
Before the temperature reaches 0 degrees, it has to reach 4 degrees first.
When the temperature reaches 4 degrees, the water will sink.
Water that reaches this temperature will continue to sink until the entire water beneath the surface of the pond is 4 degrees.
Since now all the water that has a temperature of 4 degrees is beneath the surface, further cooling can take place on the surface.
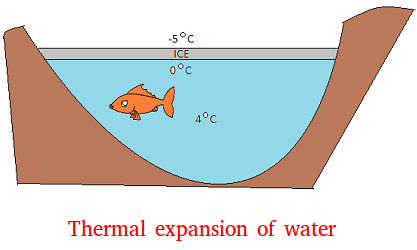
As you can see, the temperature beneath the surface is a steady 4 degrees Celsius. Suppose for example that water was densest or heaviest at 0 degree. When the ice is formed, it will just go to the bottom of the pond. The ice will just keep piling up until the entire pond turns into ice.
If water did behave that way, the fish will freeze to death.